![]()
On the Instability of Amalgams
- Increased mercury emission...
![]()
The symbiosis between the dental and industrial communities and their scientific journals
![]()
On the Instability of Amalgams...cont. -
Formation of droplets on the surface of non-gamma-two amalgams
When subjected to wear/polishing modern non-gamma-two amalgams exhibit a most striking and visual behavior of instability. Also in aged amalgams polished under cold water droplets start forming on the surface.
The deposits are concentrated to alloy particles unaffected by mercury. In the admixed Dispersalloy-type of amalgams droplets are formed on the spherical Ag/Cu-eutectic particles. These are surrounded by a halo-like ring effected by mercury containing a mix of h'-phase and g1-phase. Droplets start forming just inside this ring and continue until the entire surface of the particle is covered. Also in the single-melt type it is the unaffected alloy particles that get covered.
The first investigators to report on this phenomenon were Rehberg and Scharschmidt (30). Others later confirmed these findings (30, 31, 32, 33, 34, 35). It is noteworthy that all these papers are IADR Abstracts and not ordinary scientific articles. The formation of droplets has attracted very little attention from dental science over the years.
Droplets can be seen by the knowledgeable observer in photographs in scientific literature and even brochures used in the promotion of non-gamma-two amalgams (4, 36, 37). It is indeed hard to understand why this extraordinary and highly visible phenomenon has not been the subject of one single scientific article - as far as I can find. I have been told that some individuals in the dental establishment have regarded these droplets as polishing artifacts. I will later in this web-document prove them wrong - it is linked to an increase in the emission of mercury vapor.
In scientific articles I have only found this striking behavior of instability mentioned twice. Pleva presents photographs of the phenomenon taken in a Scanning Electron Microscope, SEM, and discusses it in some detail (38). It is also mentioned in one sentence by Herø and Jørgensen in a paper dealing with a different subject (39).
In 1986 the author of this web-document had a filling placed in a SEM. At the time I had no knowledge of the previously published IADR Abstracts and indeed no interest in the amalgam-issue either. This particular amalgam was a filling taken directly from the mouth without being subjected to any polishing before the experiment. On the surface there were some small droplets that caught the attention of the SEM operator. The droplets were observed in a place of suspected compressive stress generated by the screws holding the filling in place.
The hypothesis that compressive stress was responsible for squeezing mercury out of the filling was tested. A special equipment for use under vacuum in the SEM was designed and manufactured. A number of Delrin® cylinders with cylindrical holes, Ø3x5 mm were manufactured. These holes were filled with the amalgams Dispersalloy, ANA 2000, Ardent Futura and Royal by a dentist using clinical standards. After ageing several months the cylinders were polished with wet grinding-paper down to grit 4000. The fillings were put into the SEM and subjected to a well-defined compressive stress.
The hypothesis was proven wrong. The droplets appeared despite the presence of compressive stress or not. The specimens could not even withstand being polished under cold water.
A second hypothesis was formed. It was presumed that mercury was so loosely bound in non-gamma-two amalgam that Hg travelled rapidly and freely as result of surface diffusion along dislocations in the surface and below.
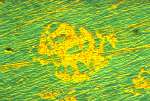
It was found that the droplets were easily observed in an ordinary metallographic, visible light microscope, see Photo 3. The following experiment was devised:
A specimen of Dispersalloy® was polished at 07.12 Mars 1, 1986. It was put under the microscope and an area with emerging droplets was selected for investigation. The computerized camera was started at 08.18. The first ten photos were taken with an interval of ten minutes, the next twenty photos were taken every twenty minute and finally forty photos every forty minute. This strategy was chosen because the formation of droplets was fastest in the beginning and then slowed down. The total sequence of 70 photos took 35 hours.
To get maximum visibility a technique utilizing polarized light was chosen. It was found that when using interference contrast according to Nomarski the droplets showed up bright yellow on a green background surface. The successive growth can be seen in Photo 4, Photo 5 and photo 6 all taken in polarized light. Photo 7 shows the investigated area in visible light after the completion of the photo-sequence.
Photo 4 Photo 5 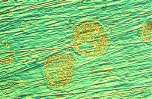

Photo 6 Photo 7 Click in the image to view full size!

The movie!
Click in the image
to view movieAn animation of the formation of droplets on the surface of Dispersalloy ® can be seen in Movie 1 (Warning: 671 kbytes!).
The photos were sent to The National Board of Social Health and Welfare, SoS, who financed a small pilot study at the Swedish Institute for Metals Research (40). A number of droplets were removed from the surface and five had their composition analyzed. Four of them had a mercury content ranging from 44.1 % to 49.6 %. The fifth contained 85.4 % of mercury. The investigator concluded that a further investigation was necessary to explain the mercury values obtained. I immediately replied that on good grounds it could be suspected that the technique used - extraction replica - only striped the solid particles being unable to deal with a thin mercury film on the surface. No further investigation took place, however. SoS regarded this financing as an extraordinary situation since they do not normally finance scientific research.
A similar photo-sequence of a single-melt non-gamma-two amalgam (ANA 2000®) can be seen in Photo 8, Photo 9, Photo 10 and Photo 11. The principles for the formation of deposits on the surfaces of modern non-gamma-two amalgams can be seen in Figure 4 and Figure 5.
Photo 8 Photo 9 Photo 10 Photo 11 Figure 4 Figure 5 Click in the image to view full size!
Photo 12 Another phenomenon not previously shown is the formation of bands of droplets, see Photo 12. They were created by making one big scratch on the surface and then grinding it away until it was invisible. Probably the many dislocations appearing beneath the scratch provided pathways for a heavy diffusion.
Photo 13 Click in the image to view full size! On rare occasions single droplets could be observed on conventional amalgams but in the majority of tests there were none. At the edge of the sample directed away from the grinding action a small band of severely deformed metal extended over the plastic cylinder in which the amalgam was contained. This band was often covered with a mass of deposits, see Photo 13.
To Increased mercury vapor emission from modern non-gamma-two amalgams
On Reality. Publisher and editor: Bo Walhjalt. ISSN 1650-9323.
© Bo Walhjalt and authors. | Comments on this page
Latest update 2002-12-05original url of this page: http://www.gbg.bonet.se/bwf/art/instab/formation.html